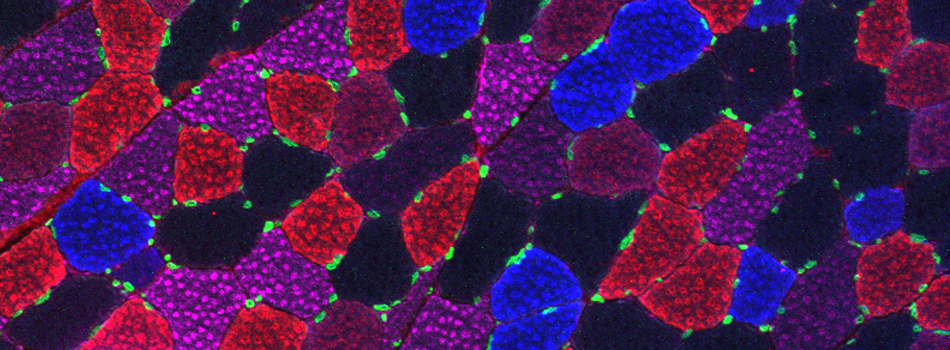
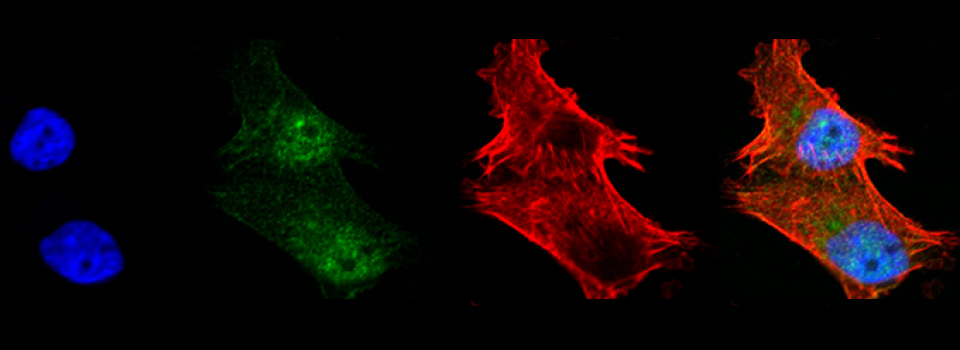
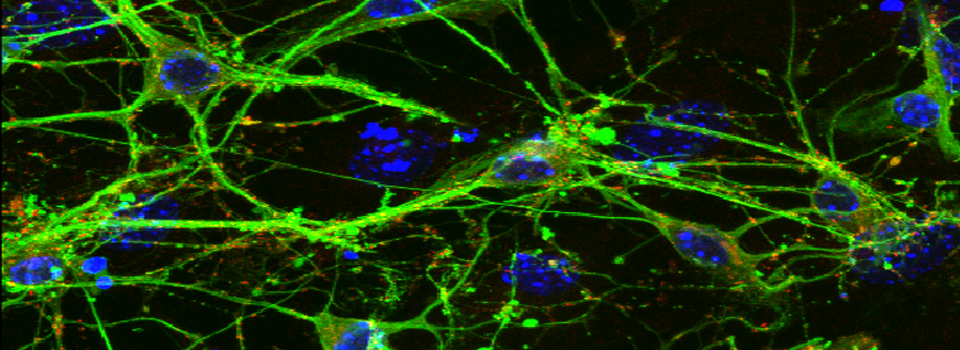
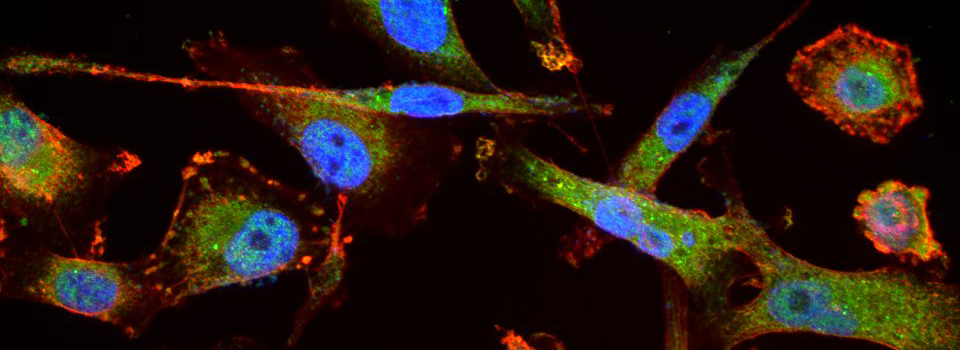
FV3000- Basic Microscope Operation
Olympus FV3000: Basic Microscope Operation
(How to get a specimen on the stage and find something worth imaging)
Important note- the FV3000 is an inverted microscope, which means people will use a wide variety of containers for their specimens. There is also the option of using the stage top incubator, which puts specimens up even higher. What this means is that the focal plane can and will be all over the place, and you should be prepared to take some time to find it for your specimen.
I. Controlling XYZ movement
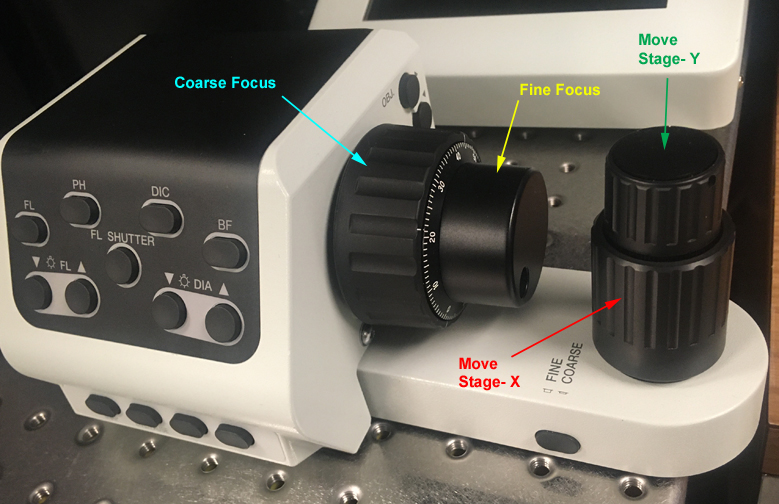
These controls are on the right side of the air table. Turning the focus knobs towards you raises the objectives (towards the specimen); turning them away from you lowers the objectives. When using oil objectives with short working distances, you should be primarily using the fine focus knob. The X-Y knobs should be exclusively used to move the stage.
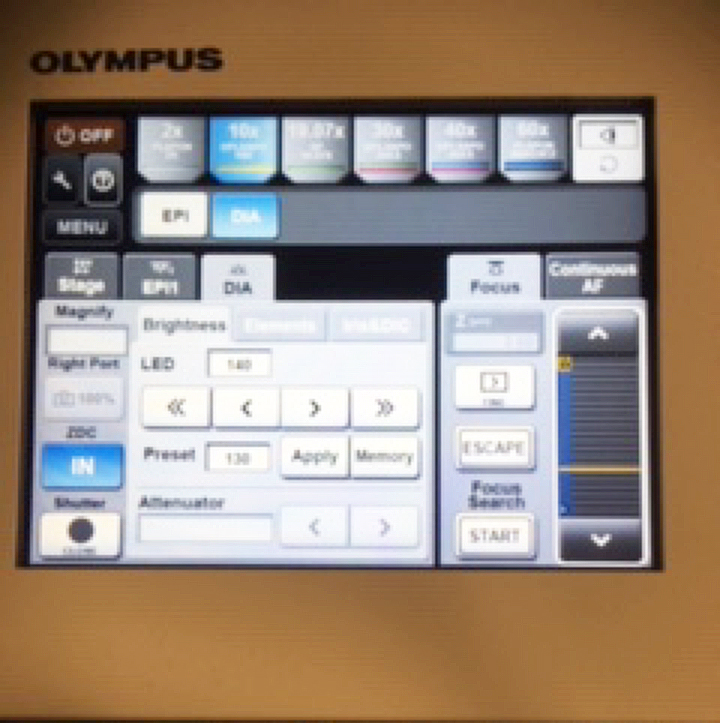
II. Control pad (Box #3)
This device controls the view mode (eye pieces or confocal), the form of illumination for the eye pieces (Epi-fluorescence or DIC bright field), the objectives, and allows you to record 8 separate x-y coordinates so that you may send the stage to regions of interest.
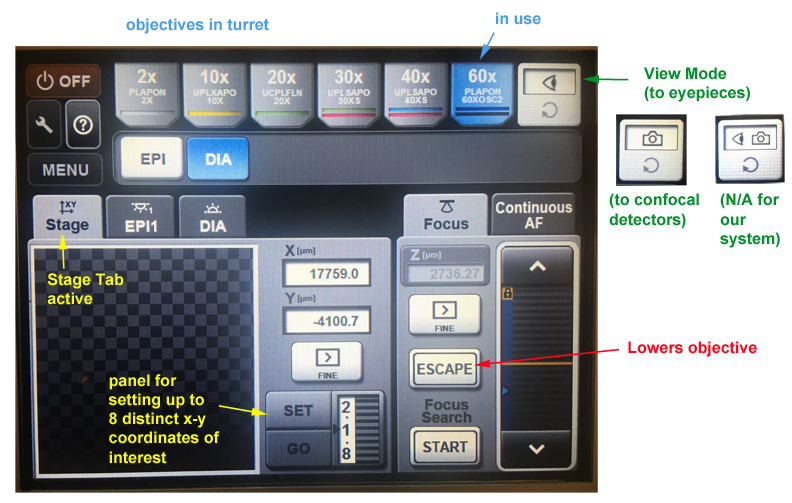
The “Escape” button lowers the objective turret, which is needed for the use of oil objectives (covered in detail later).
The “Focus Search” function is a nice shortcut that finds the coverslip. If the device successfully fines the coverslip, you will hear one beep (good). If it can’t you will hear 3 beeps (bad). If it fails, the most likely cause is a previous user who used a very different specimen container and shifted the focal plane. Your best bet is to go to the 2x objective to focus on the edge of a coverslip or well, then switch back to the 10x to find your target. You can then reset the Z origin and the Z-limit (if needed) in the Fluoview software. The search function will also fail if the ZDC DM is “Out” instead of “In” (“Microscope” Tab in the Fluoview software).
The “EPI” tab allows you to control the filter cube:
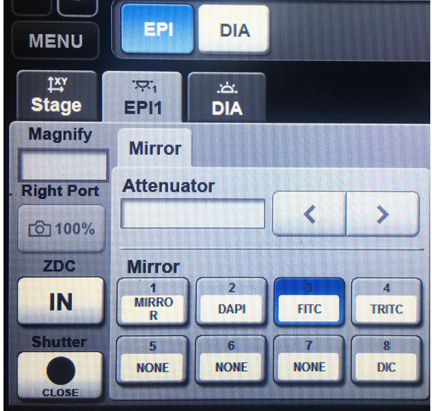
There are 3 filters available: DAPI, FITC (green/yellow fluors), and TRITC (orange/red fluors). There are no filters for viewing dyes that fluoresce in the 600+ nm range, but you will be able to see them in confocal mode with the 640 nm laser.
The “DIA” screen option is most commonly used to adjust the light intensity.
III. Stage inserts
You have a choice of 3 stage inserts for your fixed specimens: 1) the well plate holder, 2) the universal slide holder (which you can use for a single slide or a 60mm or smaller dish), and 3) the 4-slide holder. Remember to place your slide coverslip down. If you use a plastic vessel, you are limited to the air objectives (2x, 10x, and 20x). If you want to use an oil objective, you must use a glass bottom or cover slip of the proper thickness (~170 uM).

IV. The Tokai Hit stage enclosure

Our system includes a stage enclosure because our room is drafty and we cannot control the thermostat. For fixed specimens you can leave the front hatch up. For a time-lapse scan of any great length, you have the option to put this lid down to keep the stage environment more stable. This enclosure does introduce an extra step in tilting back the condenser (which you need to do when placing stage inserts or oiling/ wiping off objectives).
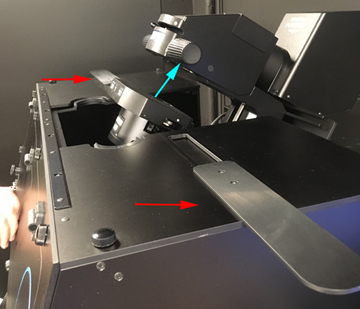
To open the space around the condenser, push the two sliders (red arrows) outwards. You can then push up and back on the condenser focus knobs to tilt it back. You can leave the top open in you don’t need to keep drafts off the stage. The one downside of this arrangement is that it is very easy to forget to tilt the condenser back. If you don’t get an image in confocal mode, the first thing you should do is glance left to check if you see this. The lasers will be blocked unless the condenser is down.
V. Switching from Air to Oil Objectives
An inverted microscope allows you to image specimens in well plates or dishes or well slides with higher N/A oil objectives by putting those objectives underneath, so that oil does not contaminate the specimen. The downside is that oiling the objectives is trickier than it is for an upright system. The oil drop in an inverted configuration will be perched a bit precariously on the tip of the objective lens, and it needs to stay between the lens and the coverslip/ dish bottom. Too little oil, and you won’t have a proper path for the light. Too much oil, and it will start to run down the sides of the objective once it makes contact with the other piece of glass. Getting that usable blob of oil between the pieces of glass is an art form, and even experienced users may have to try several times. Once you get it placed properly, you can move the stage around within the limits of your oil blob and take multiple images. But when you have to image beyond the range of your current oil blob, or you’re changing specimens, you will need to wipe all the oil off the objective with the lens paper, and apply a new drop. It’s a hassle, but necessary, because too much oil flows down, which is bad for multiple reasons.
If you are using a well plate, you will need to pre-oil the oil objective you plan to use before you put your plate into the stage.

We have 2 types of oil: the blue bottle (ne = 1.518) for the 60x objective, and the green bottle Silicone oil (ne = 1.406) for the 30x and 40x Si objectives.
Use 1 drop for the 60x
2 drops for the 40x
3 drops for the 30x

A few more details on Objective switching
There is a button at the bottom left of the stage enclosure that will switch on a light to illuminate that space, which makes it easier to see for oiling objectives. Remember to switch it back off before you start imaging. The knob next to it controls the brightness.

The universal stage insert is the easiest to use when oiling objectives, as there is open space all around your specimen holder to use for access to the objective. With the 4-slide insert there is enough open space on the left or right side to use, even if you mount 4 slides. If you are using a well plate or the stage top incubator, you will need to pre-oil your objective, then switch to the 2x or 10x before you put your specimen/ the chamber into the stage opening.
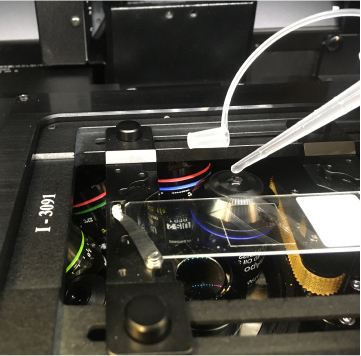
When an objective is “escaped”, do not touch any focus knobs. If you do, it will “un-escape” and move back up. The point of using this function is to prevent collisions between the objectives and the stage insert. The Fluoview software will be greyed-out and inactive while an objective is Escaped.
To remove oil from your slide, blot with lens paper (in the box on the computer desk), and then wipe the rest off with a bit of ethanol. DO NOT use Kimwipes on objectives or anything (like slide or dishes) that will be near objectives. Your specimen holders should be free of oil when you bring them into the Core, especially if you have previously viewed them with oil on a different brand of microscope. Each microscope manufacturer has their own version of immersion oils, with different refractive indices, so they are not compatible.