Events
An Opportunity to try the Nikon NSPARC Super-Resolution Microscope

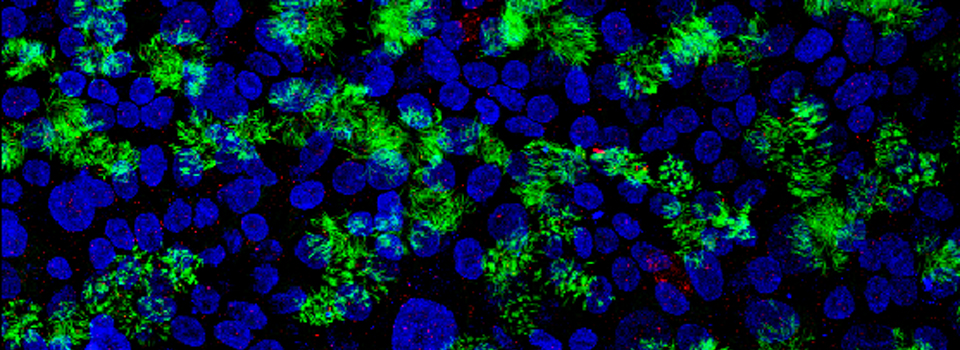
Coming October 1-10, 2024: TomoCube Demo in the BBIC
This is your chance to try an exciting new imaging technology for FREE! People with Booked accounts should e-mail the Core manager for access to the Demo on the scheduling calendar.
10-8-20: SOON

10-2-20: It’s like Christmas time in the BBIC

9-24-20: THE FV3000 HAS LANDED!!!!
Despite pandemic, NIH suspense, and another round of floods, the Olympus FV3000 has completed its journey to SR2!

We expect one more big box next week, then on to Step2: putting it together. We will keep you updated.
6-16-2020 NIH S10 GRANT APPROVED!!!!
The BBIC is thrilled to announce that we will be purchasing a new Olympus FV3000 inverted confocal microscope. The exact timeline TBA, but check back here for updates. Rough guess for installation is late August/ early September 2020.
UPCOMING DEMO:Olympus FV3000 at the BBIC
WEDS. MARCH 28, SR2 Room 401, Seminar by Eric Bridenbaugh of Olympus
MON, April 16 - THURS, April 29, Live demonstrations in the BBIC (SR2 328A)
This demo will include a stage top incubator for time lapse imaging of live cells.
Nikon SMZ-25 Research Stereo Microscope demo at the BBIC

This instrument was at the BBIC for hands on testing March 8-12. Its features include: motorized focus for automated Z-stack acquisition, motorized fluorescence, motorized zoom, oblique contrast LED illumination, wide magnification range: 3X-315X, high resolution optics.
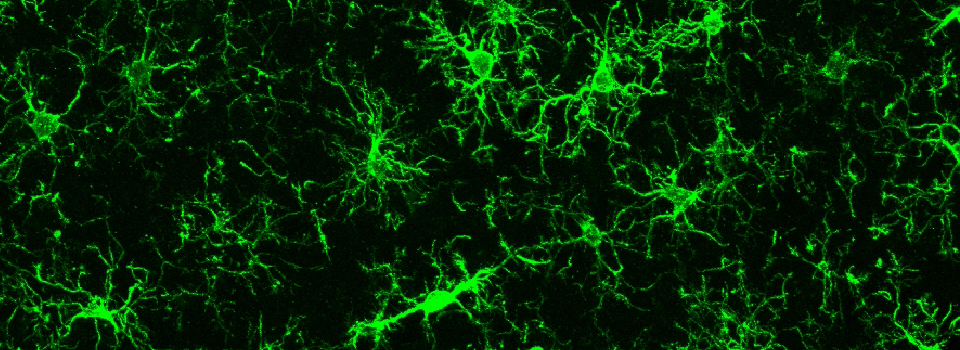
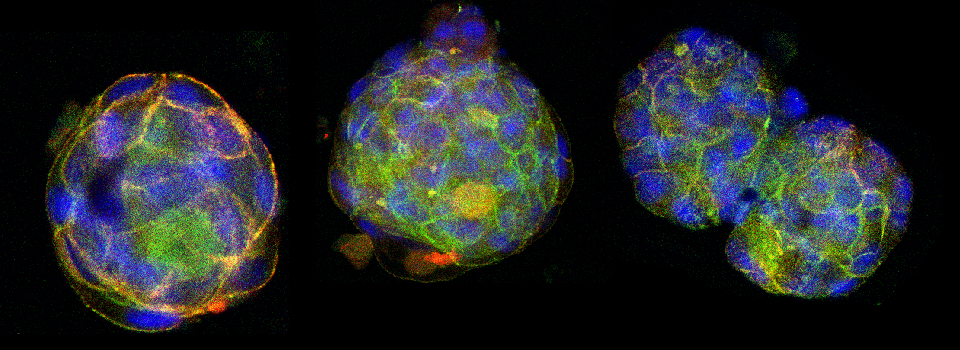
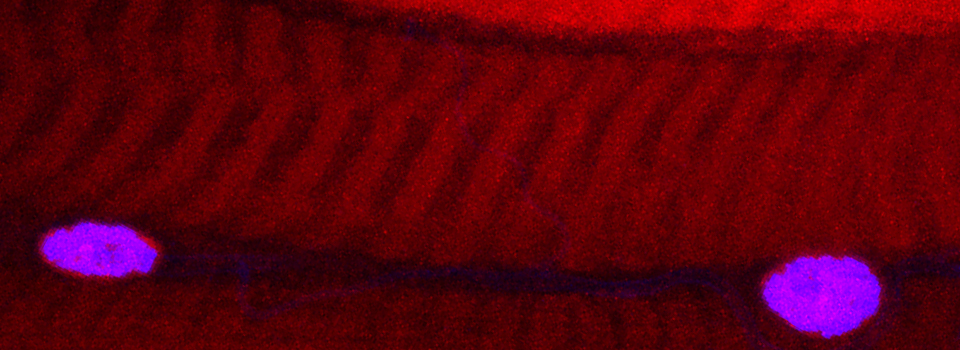
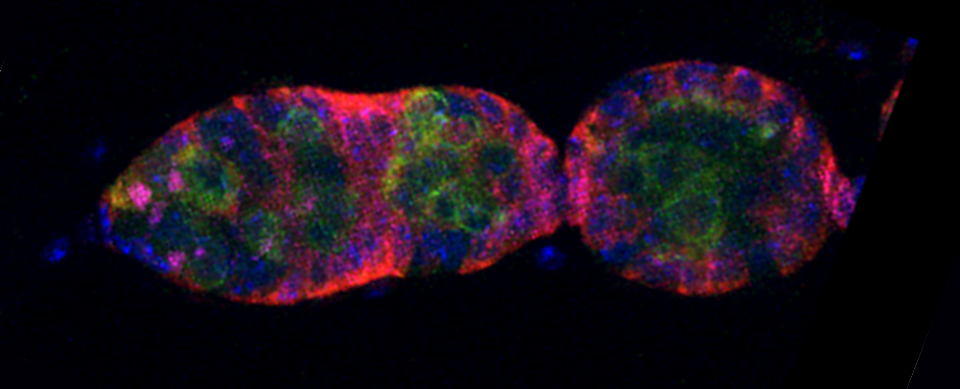
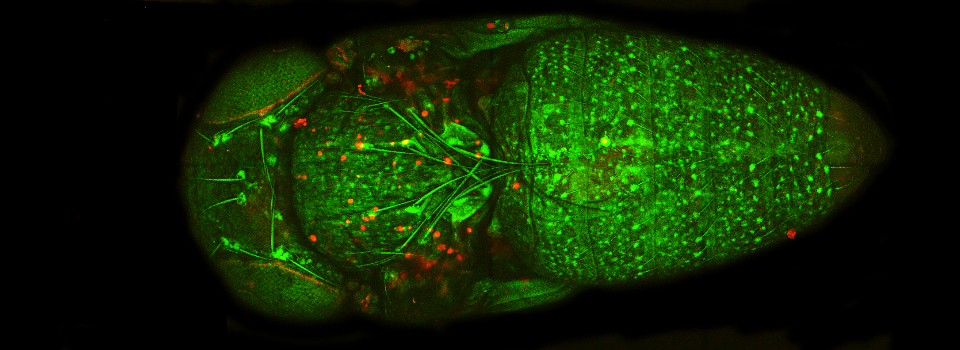
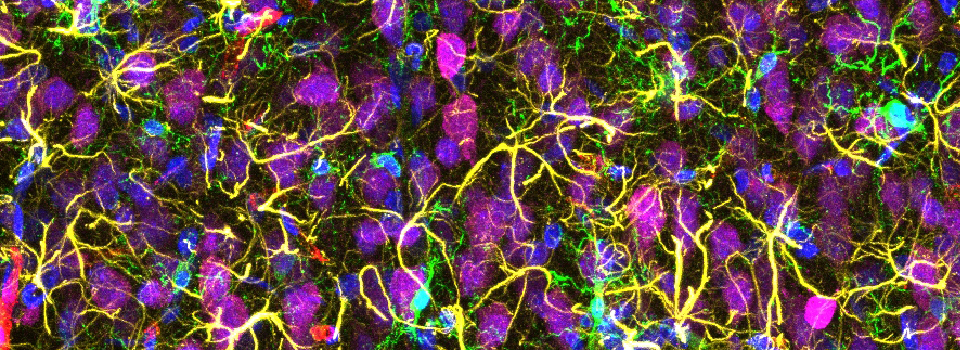
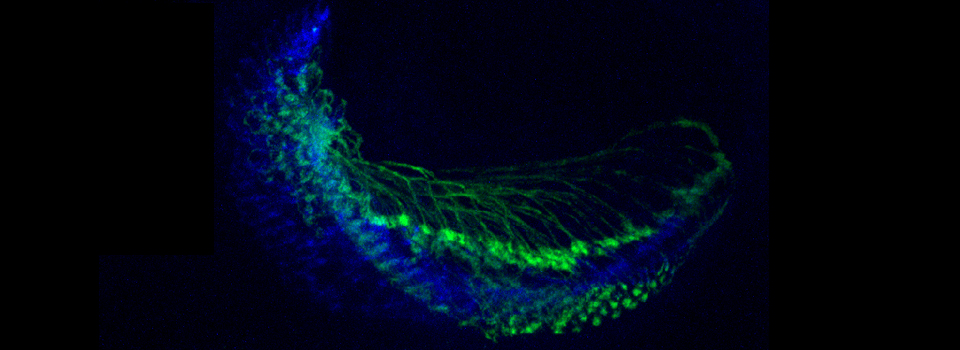
Some images from the Demo:
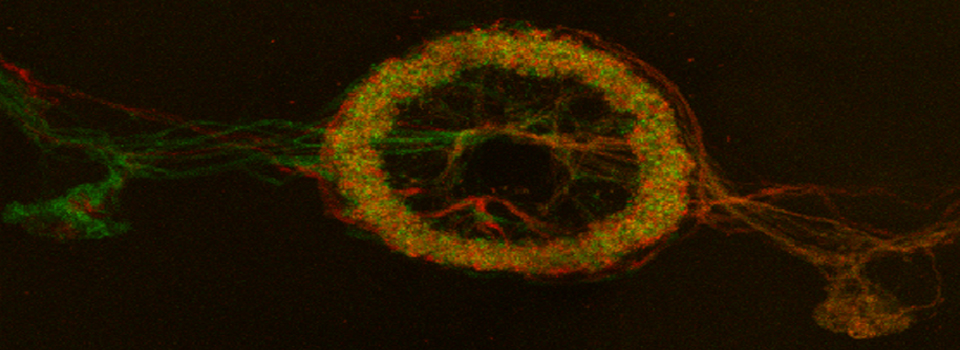
An early Drosophila pupa expressing actin-GFP and esg>Ds Red
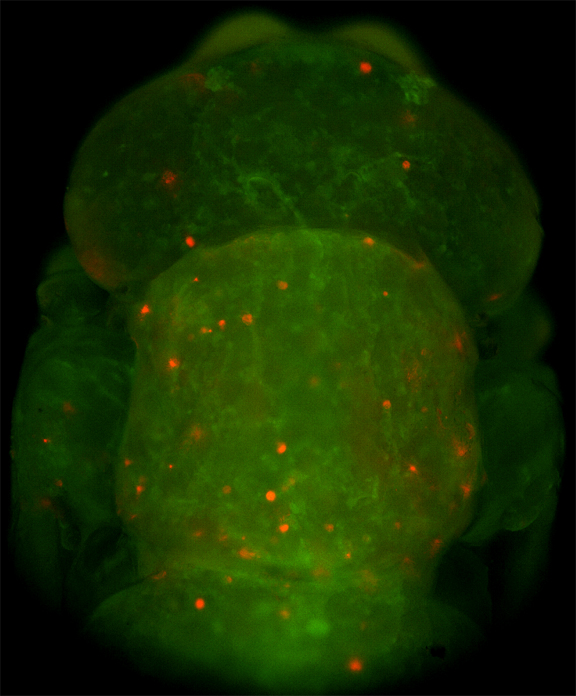
In situ hybridizations to zebra fish embryos
This instrument can also do time lapse imaging (which I could have used during my postdoc-it would have spared me some all-nighters!). Here is a movie of a Drosophila expressing mhc-RFP undergoing the larval to pupal molt; the animal is detaching itself from the larval cuticle, which is hardening into the pupal case.
(movie embedding in progress)
Zeiss Airyscan Confocal Demo at BCM
The Baylor Optical Imaging and Vital Microscopy Core hosted a demo of the Zeiss Airyscan confocal in February 2018. This system uses a novel type of detector, with many small pinholes. It reminds me very much of a Drosophila eye:
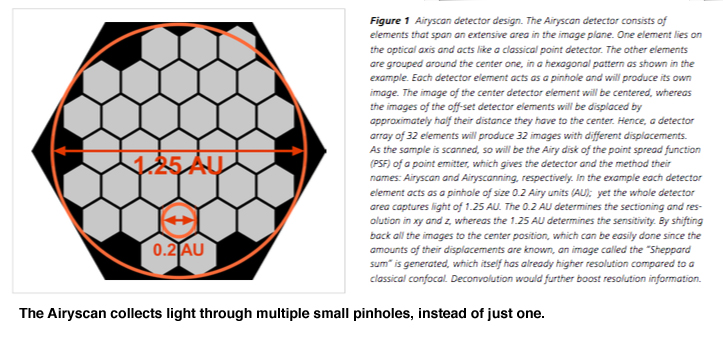
This configuration is advertised as increasing the sensitivity, resolution, and speed of the data collection, and improving the signal to noise ratio by 4-8 times!
I had an appropriate slide to test some of those claims- a triple stained preparation of a Drosophila ovary that had been accidentally left out in a lighted room, causing the green and red fluors to degrade. Looking through the eyepieces, I could see that the DAPI staining was still strong and sharply defined. I could still see reasonably strong green fluorescence in the cytoplasm, but the background was high (which often happens when fluors degrade). I could not see any red fluor at all. Could the Airyscan extract any data?
Here are the results:
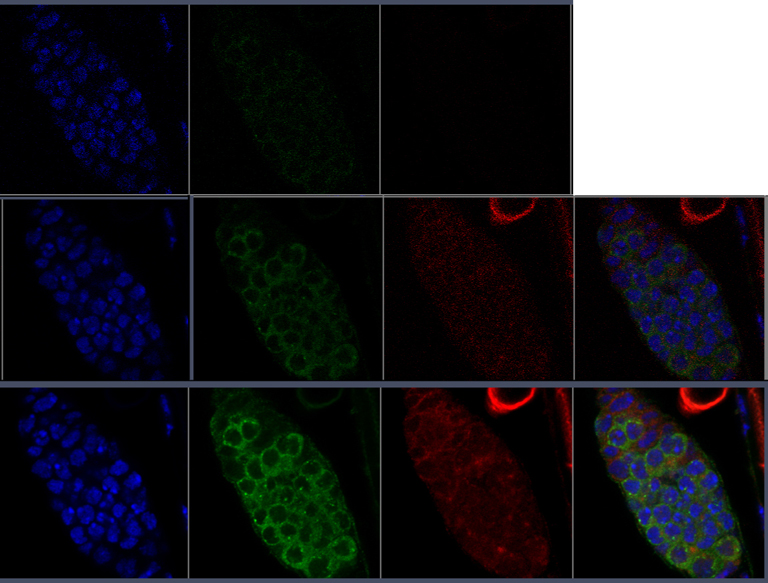
This first row shows the raw data, and in the next two rows you can see how the software combining the light collected from each individual pinhole and doing deconvolution manages to extract a very good image from a specimen that initially appeared to have no signal in the red channel, and a very poor signal in the green channel. This is most impressive.
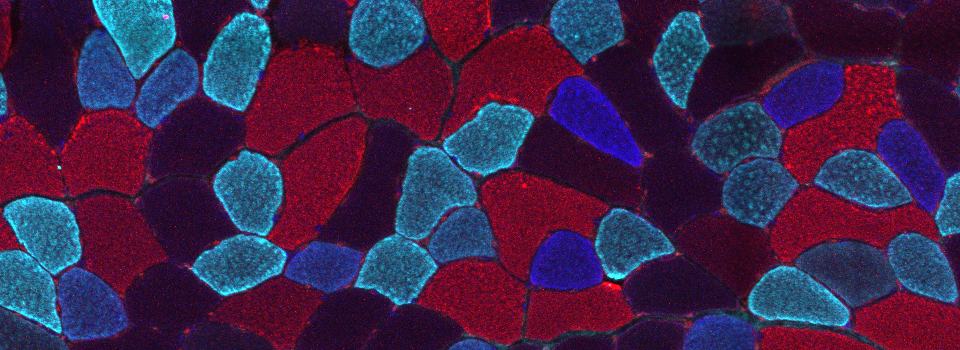
Olympus FV3000 demo at the Methodist Research Institute
On Monday 8/15/16 I tested out Olympus’ latest confocal microscope. The biggest change is in the software package, which allows for greater flexibility and customization in your scan settings. I had the chance to try out some types of objectives that we don’t currently have.
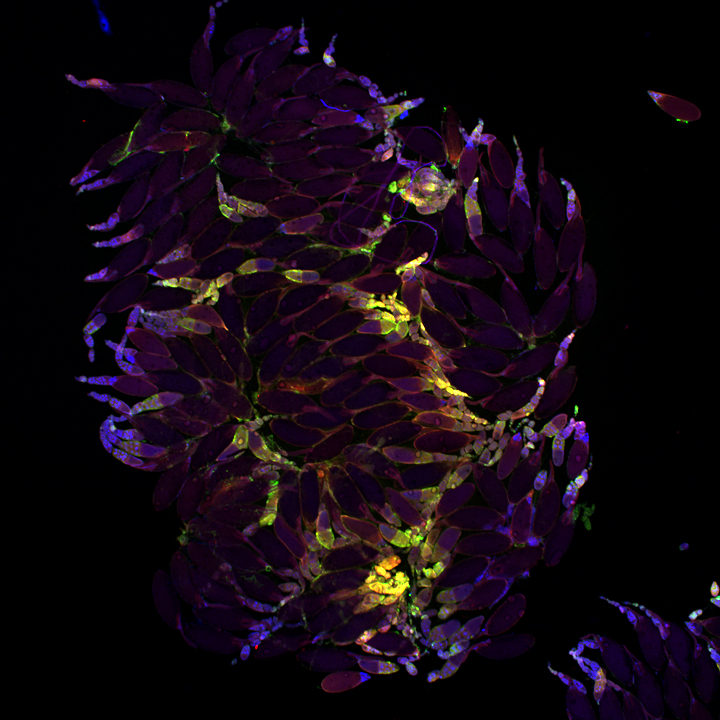
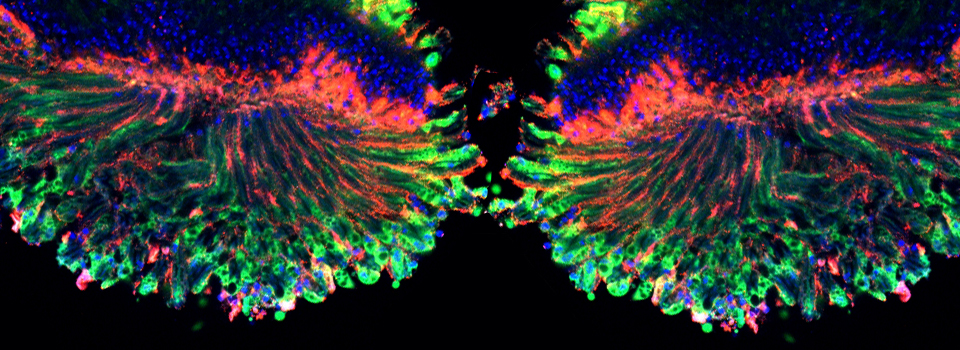
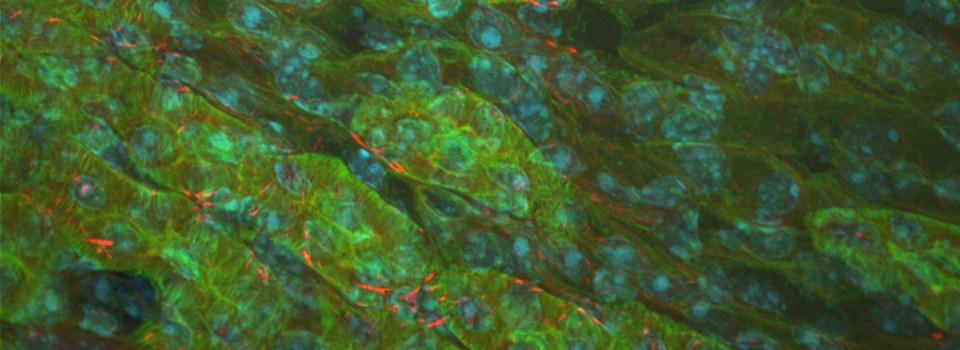
This is a 3-channel image of a Drosophila ovary taken with a 2x objective. The detail is very good- you can clearly see the nuclei in the mid-stage egg chambers. This wide view makes an excellent roadmap for finding points of interest to zoom in on: click on the region, and the motorized stage will place it in the center of the field of view. Then you can switch to the oil objectives. The slide holder allows you the room to put oil on an objective without having to remove your slide- very convenient!
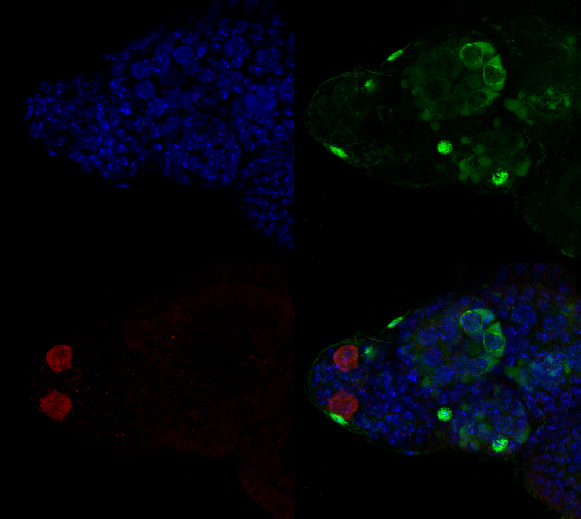
This is a very closeup image of a germarium/ early stage egg chambers using the 100x oil objective and a zoom of 1.5.
![]()
![]()
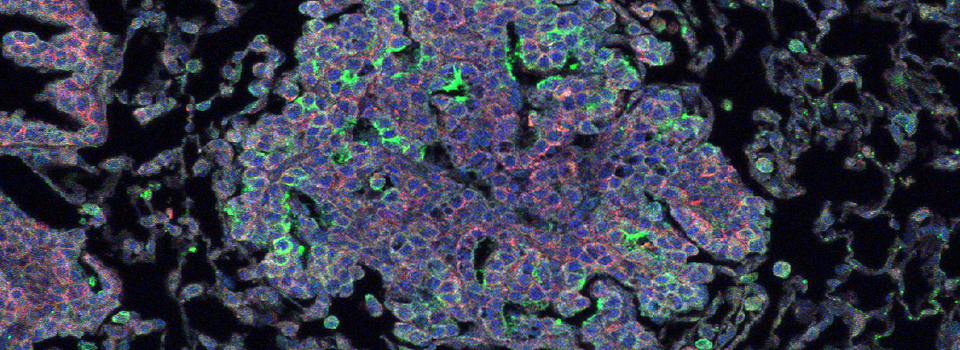
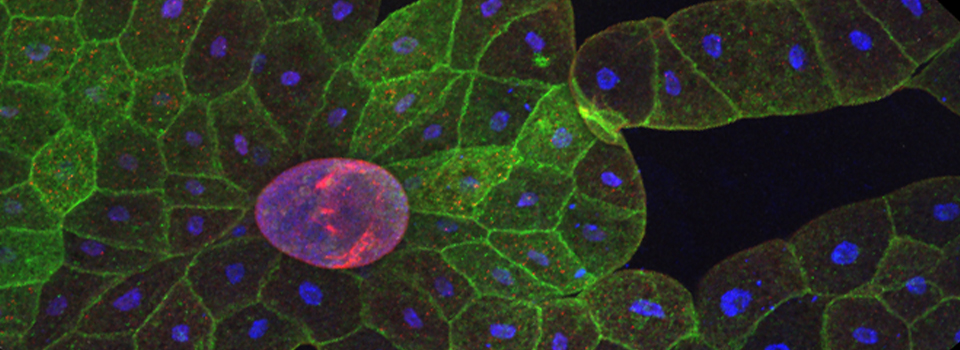
These germaria images were taken using the 60x silicon oil objective and a zoom factor of 4. This type of objective is ideal for thicker specimens mounted in liquid media (in this case Vectashield) because the silicon oil has a lower refractive index (1.4) which is a closer match to the biological materials/ mounting medium. (The first image has 33 z-sections at 0.43 micron thickness for a total thickness of 14.19 microns, and the second has 44 slices of 0.46 micron each for a total of 20.24 microns thickness.)
Thank you to Erin Kelleher for providing specimens for the test drive!!
Coming in the Spring 2017 Semester:
“The Poetics of Microscopy”
The BBIC will be collaborating with the English Department on a creative writing project about the process of science. Any BBIC user who is interested is welcome to participate. More details to follow in the Fall 2016 Semester.
OLYMPUS STAGETOP INCUBATOR DEMO: March 15-April 3, 2016

We have an INU stagetop incubator on loan for the next 2 weeks. This system fits into the stage of the Olympus FV1000 and allows you to control temperature and CO2 levels to maintain live cells for observation via confocal microscopy. We have stage inserts for well plates, well slides, and 35 mm dishes (make sure they have glass bottoms designed for confocal use).
Science and Art at the Blaffer Gallery
“Janet Biggs: Echo of the Unknown” opens Friday, Jan 16,2015, 6pm, at the Blaffer Art Gallery. This work explores role of memory in the construction of identity, and the effects of Alzheimer’s disease. Part of the exhibit features images taken by Craig Vollert (Ericksen Lab) on the BBIC’s Leica SP8.
Optical Methods Course (BIOL6222)
Additional BioStation Training
Ned will return Jan 13-14. Anyone interested in using the BioStation may sign up for a Tuesday afternoon session (1pm-3pm) or a Wednesday morning (9am-11am) time. This will cover how to use the machine, in addition to data export and analysis.
Starting 12/11/14: Nikon BioStation Demo
The BBIC will host a demonstration of the Nikon BioStation CT,
Who should attend?
- Anyone interested in kinetic imaging of cultured cells, especially long-term experiments
- Anyone interested in high through-put screening
- Anyone working with stem cells
- Anyone who would like to try out novel uses for this instrument (such as kinetic imaging of fish or fly or frog embryos)
- Anyone with any other interest/ questions
SCHEDULE OF EVENTS
12/11/14, 10 am-12pm, SR2 301: Presentation and Q&A session about the BioStation, by Ned Jastromb.
12/11/14 1pm- Ned will visit the labs of all interested researchers, to discuss experimental design/sample prep
12/15-17 BioStation setup/ calibration
12/15/14 (afternoon) - 1/16/15 (could possibly be extended to 1/23/15, depending on user interest) BioStation available for experiments. There will be a sign up column on Quartzy, under the “Biology Imaging Core” Equipment signup page. People who are officially signed up have the rights to set the conditions (temp, [CO2], etc.) and use up to 30 available vessel slots. Other interested people may concurrently use any unclaimed vessel slots with permission of the Core Manager. The plan is to run the cell culture experiments first, then try the embryo scans towards the end of the demo.