Olympus FV3000 Inverted Confocal Microscope
IMPORTANT NOTICE FOR ALL USERS.
This instrument was acquired via an NIH S10 Instrumentation Grant (1S10OD026827-01A1). When you use this microscope, you agree to cite this grant in the acknowledgement section of all publications that include FV3000-generated data.

Objectives
- Dry objectives
- 2X PLAN APO, N/A 0.08,WD 6.2 mm
- 10X UPLSAPO10X2 N/A 0.4,WD 3.1 mm
20X UCPLFLN20X N/A 0.7, WD 0.8-1.8 mm, w/Correction Collar
Plan apochromatic oil immersion objectives
60X PLAPON60XOSC2; SUPER CORRECTED, N/A 1.4, WD 0.12 mm, BFP1
Silicone Oil immersion objectives
- 30X UPLSAPO30XS, N/A 1.05,WD 0.8 mm, w/Correction Collar
- 40X UPLSAPO40XS, N/A 1.25, WD 0.3 mm, w/Correction Collar, BFP1
The PLAN APO2x can image an entire well of a 96 well plate. The UCPLFLN20X can take images through glass bottomed AND plastic vessels. The UPLSAPO30XS has a very long working distance of 0.8 mm and is ideal for thicker specimens. The PLAPON60XOSC2 is the best color corrected objective available and is your best option for co-localization work.
Lasers
- 405 nm diode laser
- 445 nm diode laser
- 488 nm diode laser
- 514 nm diode laser
- 561 nm diode laser
- 640 nm diode laser
Key Features of our new Instrument:
Hybrid Scanner This scanner has two options: a standard galvanometer ideal for slower and more precise scans, and a resonant scanner for high speed scans. The resonant scanner can scan up to 30 frames per second without a forced reduction of the field of view. The FOV is the same as that allowed by the galvanomic scanner (FOV18 at 512 x 512 pixels digital resolution). It is possible to increase the speed up to a maximum of 438 frames per second with a 512 x 32 pixel field. The resonant scanner is fast enough to record high-speed biochemical events through calcium imaging or FRET. It is also ideal for imaging living specimens due to minimized photobleaching and photo-toxicity.
High Sensitivity GaAsP detectors These detectors are twice as sensitive as the standard PMT detectors. GaAsP detectors have a maximum quantum efficiency of 45% and are Peltier cooled to reduce noise, which allows for collection of high quality images under low excitation light, ideal for live specimens. Our proposed system would include one pair of GaAsP detectors and a pair of standard PMT detectors for maximum utility. The standard PMTs have a greater spectral range, extending into the far red, and are better suited to the reflectance mode used for imaging non-fluorescent specimens.
IX3-ZDC2 Z-Drift Compensation System Temperature fluctuations can cause a loss of focus during long time-lapse experiments. Addition of reagents to living specimens can also disrupt the focus. The Z-drift compensator uses minimally photo-toxic infrared light to allow the user to mark the location of the sample plane along the Z-axis. The system can be programmed to verify the focus before each scan or to check and adjust as needed at pre-set time intervals. Users can also opt to lock onto a single x-y plane to prevent drift during high-speed time-lapse imaging.
Silicone Oil Objectives The Olympus silicone oil has a lower refractive index (ne~1.40) that is close to that of living tissue (ne~1.38). The silicone oil objectives are ideal for collection of high-resolution images deep within live specimens, as this minimization of spherical aberration reduces signal distortion in the Z-axis. The 30x silicone objective has a working distance of 800 microns, which will benefit researchers working with small organisms such as zebrafish, Xenopus, and Drosophila. Silicone oil is more stable than standard immersion oil, which makes it ideal for very long time-lapse observations, or very large mosaic stitches, as it does not dry out and harden.
Stage top incubator Maintaining live cells or tissue explants for observation requires control of environmental conditions such as temperature, humidity, and concentrations of gases such as carbon dioxide and oxygen. The incubator has inserts that allow users to image with well plates, round petri dishes, or well slides. Perfusion ports permit the delivery of compounds into the specimen vessels at the time point(s) desired by the researcher. The gas mixing system will allow researchers to control the percentages of carbon dioxide (0 - 20 %) and oxygen (0 - 21 %), according to the needs of the experiment. The ability to also control oxygen levels will allow greater fidelity in replicating physiological conditions, as standard room levels are 21%, but cells within a living organism are typically exposed to 13% oxygen.

IX3-SSU scanning stage This stage allows users to easily switch between macro and micro-imaging. The lower magnification objectives can be used in conjunction with the Multi-Area-Time-Lapse option (MATL) to produce a complete reference map of a large specimen. The user can click an area of interest on the screen to center the field of view at the point, and then zoom in for more detailed imaging. Easy switching between air and oil objectives is facilitated by the ability to designate up to 8 different x, y coordinates into the stage controller, and to manually move the stage aside to access an oil port in the stage insert, and then return to the original coordinates. The mosaic stitching software permits more precise drawings around regions of interest than our current confocal setups, allowing users to avoid wasting imaging time by scanning empty space.
FV3000 software / Cellsens This fully loaded software package includes Sequence Manager (allows creation of complex customized imaging protocols), Spectral Un-mixing (can separate overlapping signals with a resolution of 2 nm), Deconvolution (can improve the resolution to up to 135 nm in the x-y plane, and 400 nm on the Z-axis), Count and Measure /Object Tracking (allows users to mark and quantify objects of interest, and track position vectors and velocities of moving objects in a 2D plane), and FRAP/FRET.
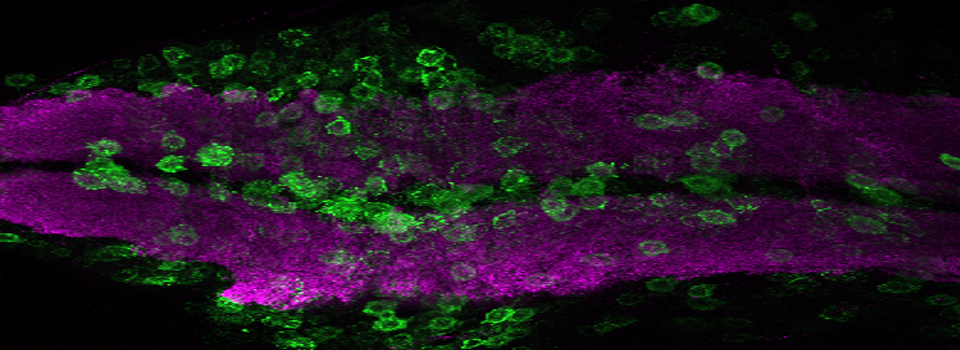
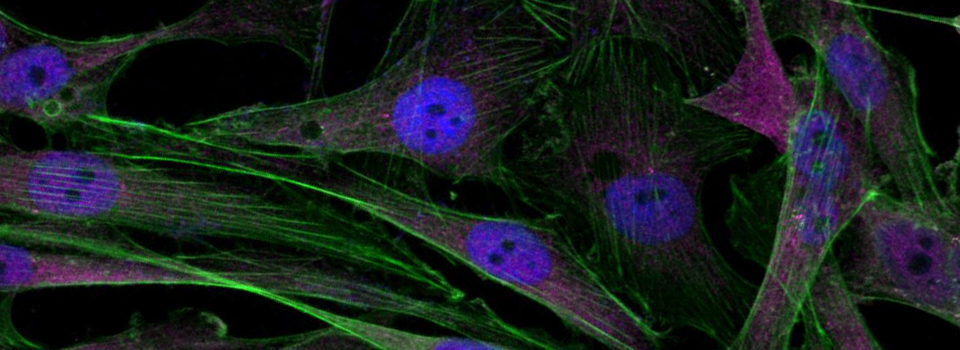
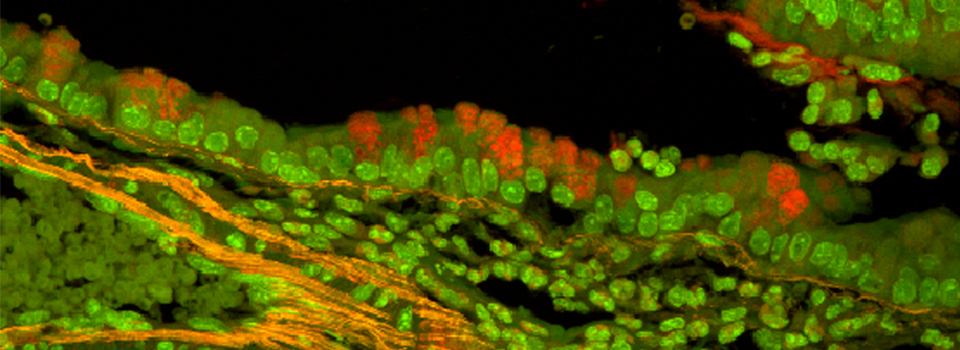
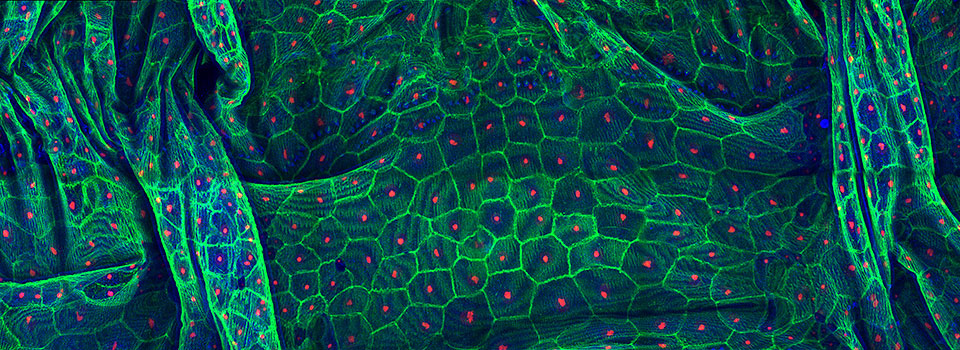
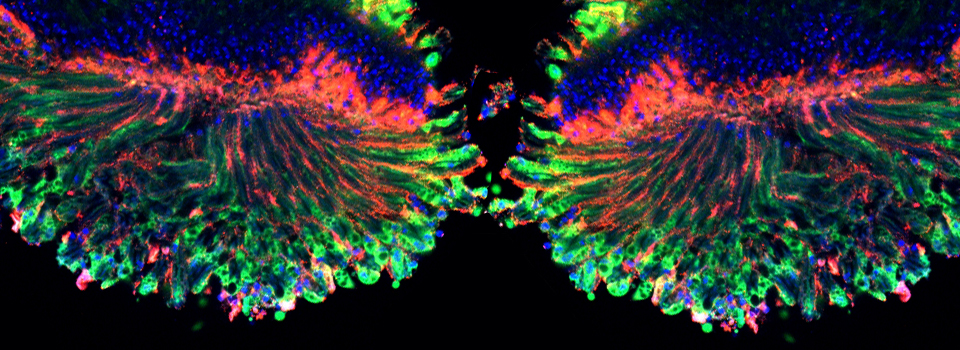
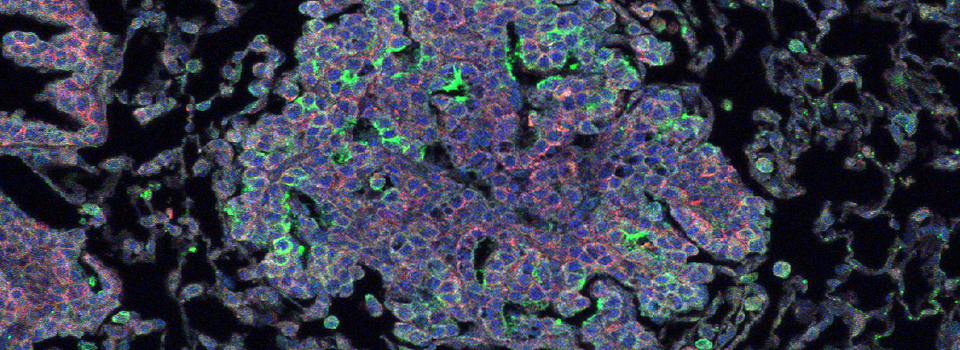
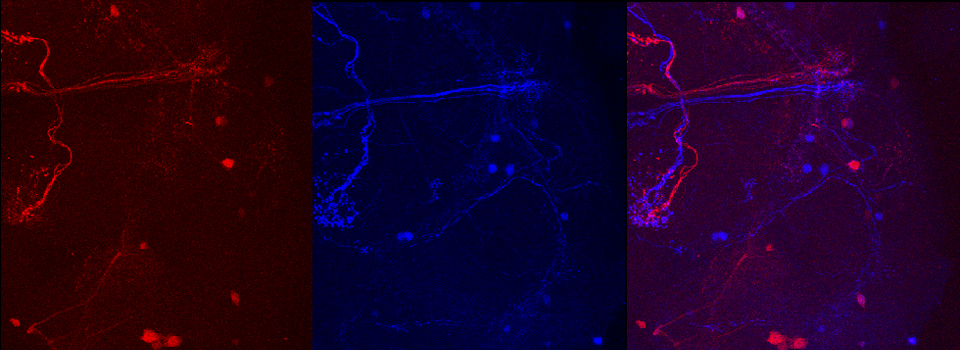
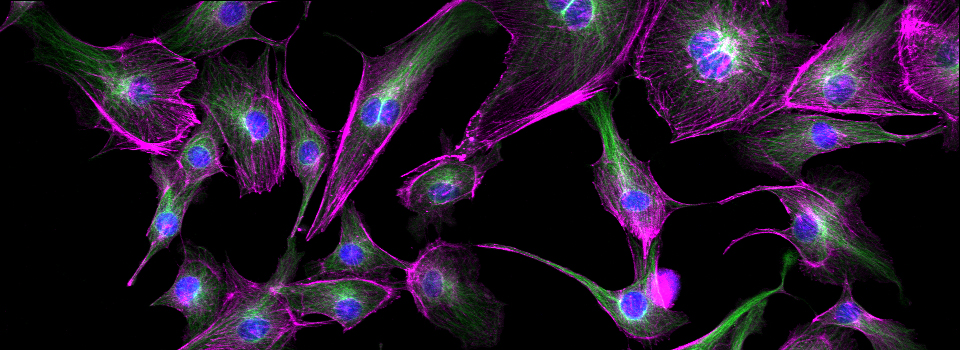
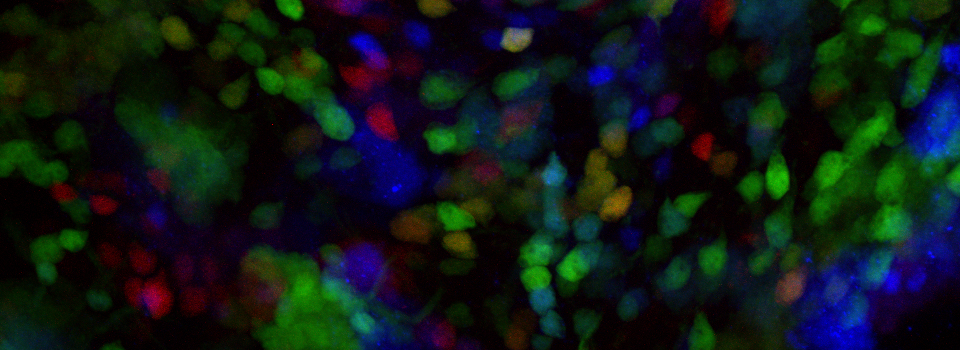
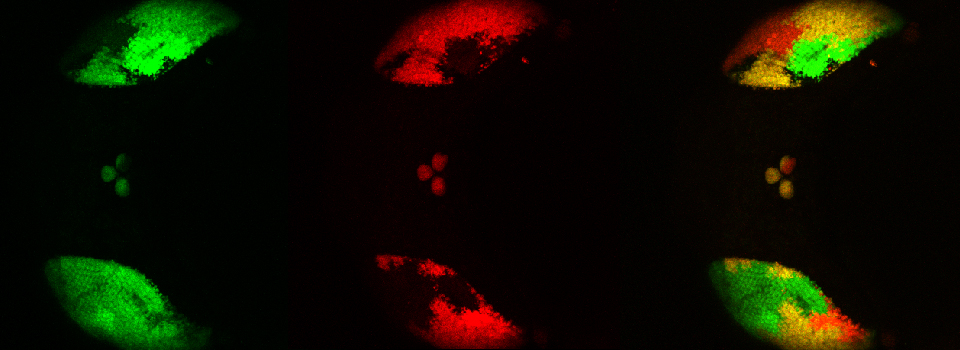
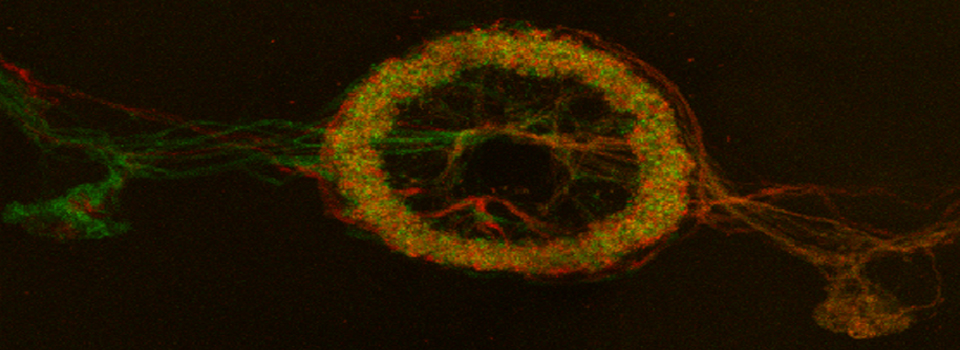
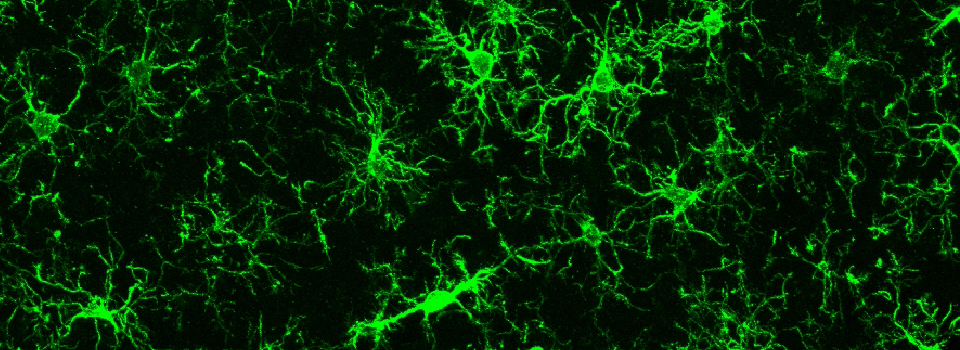
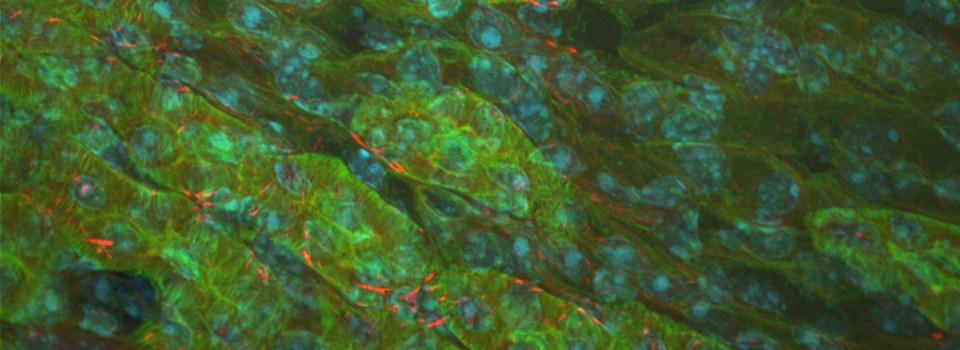
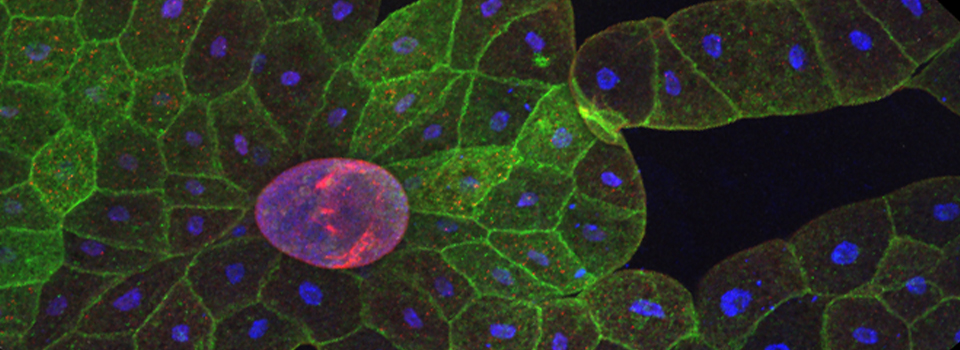
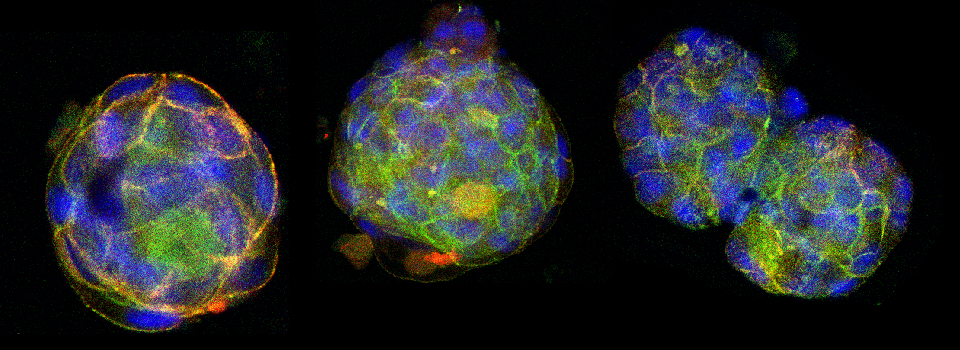
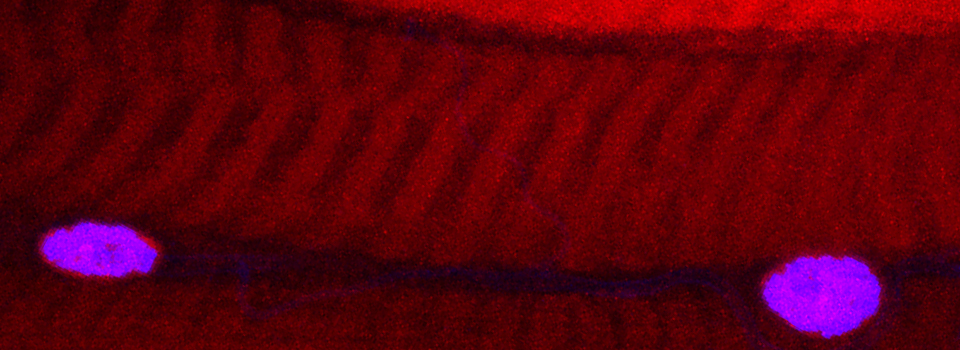
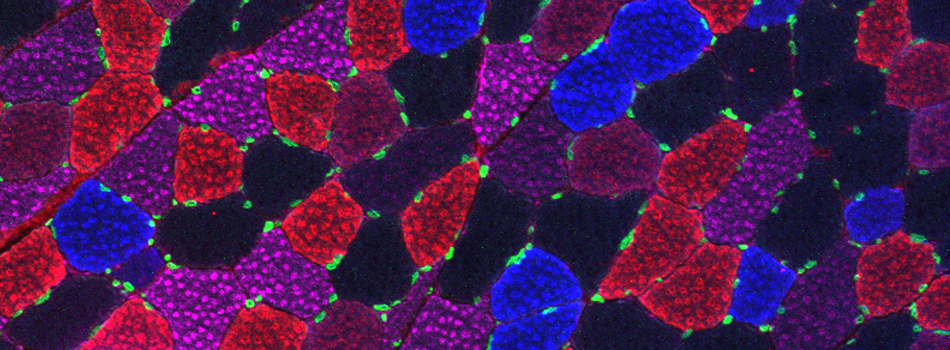
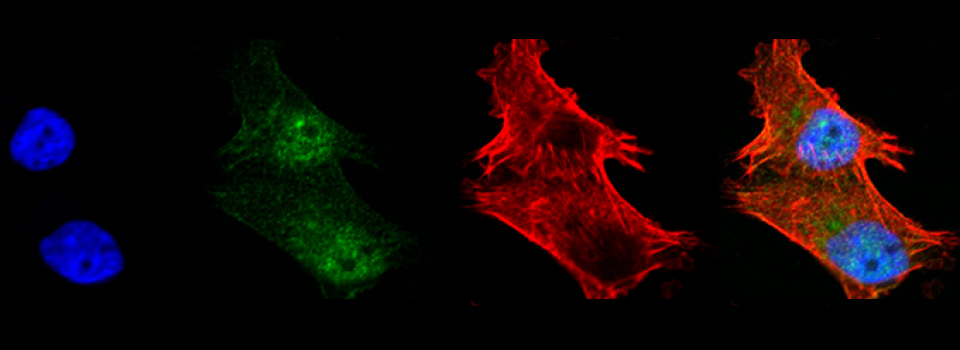
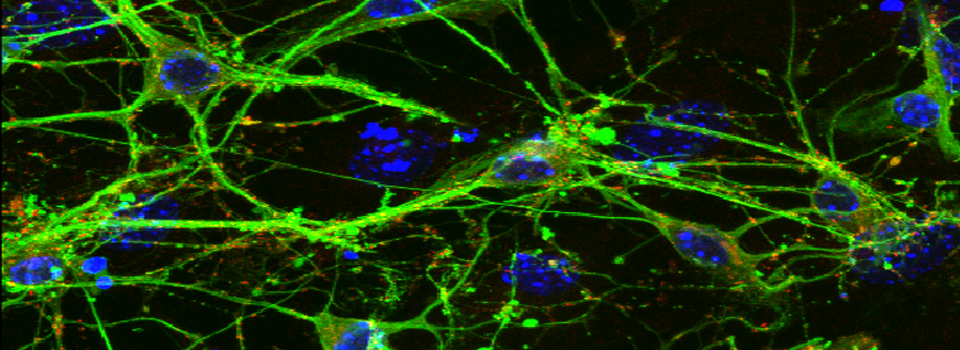
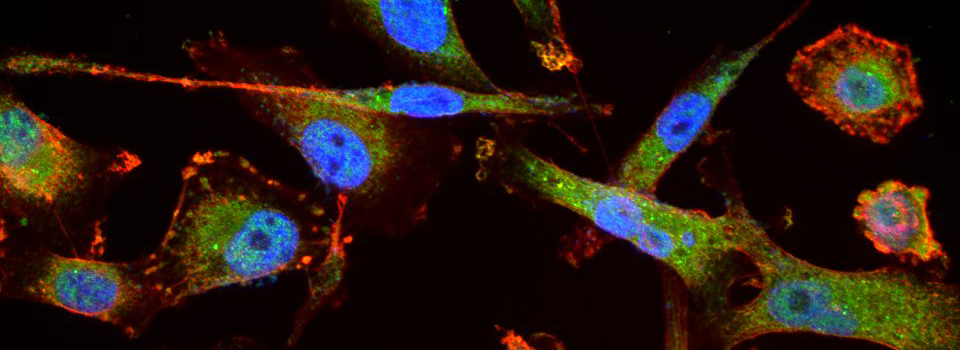
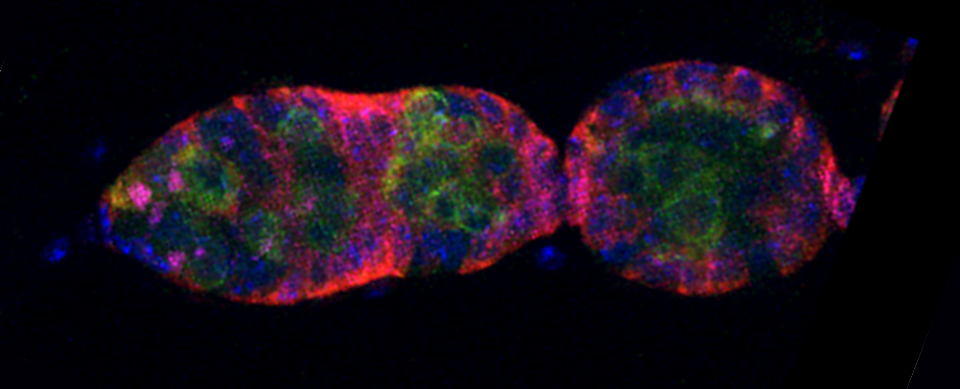
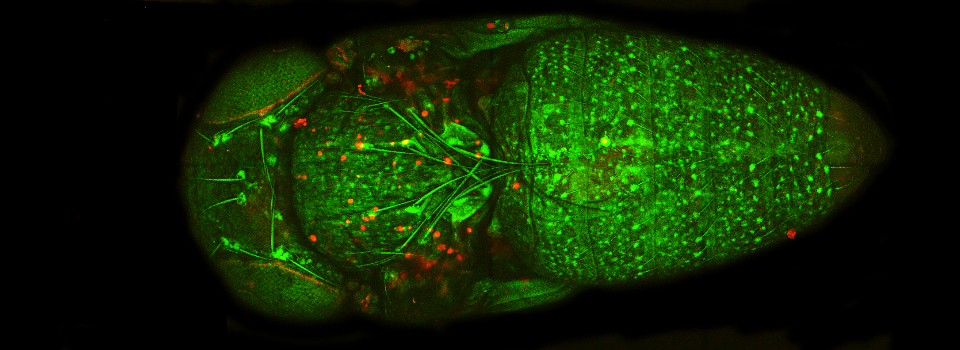
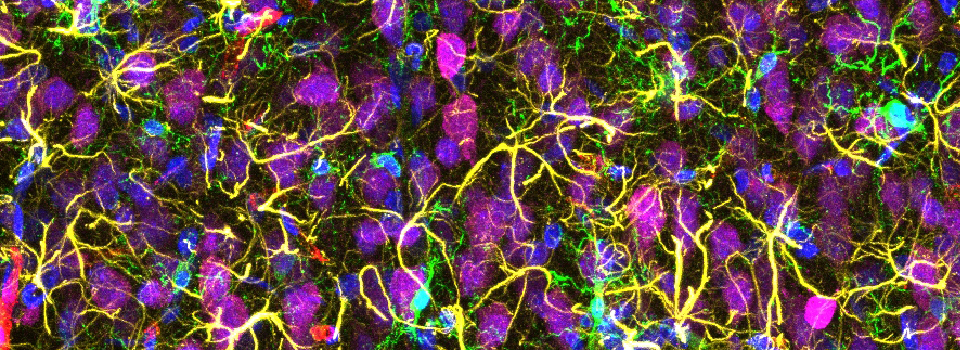
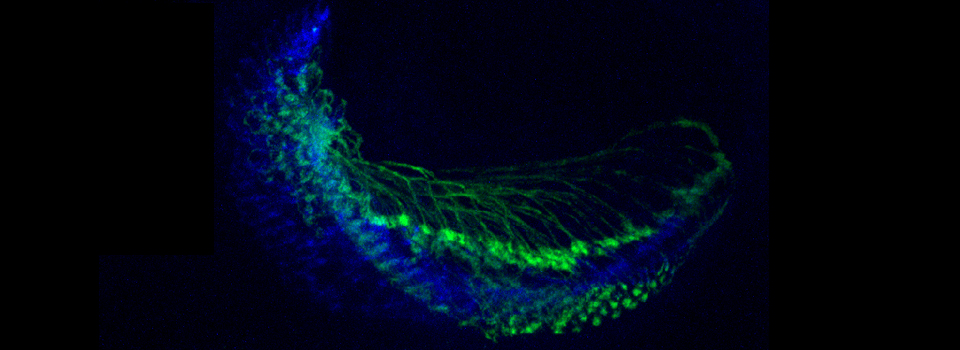
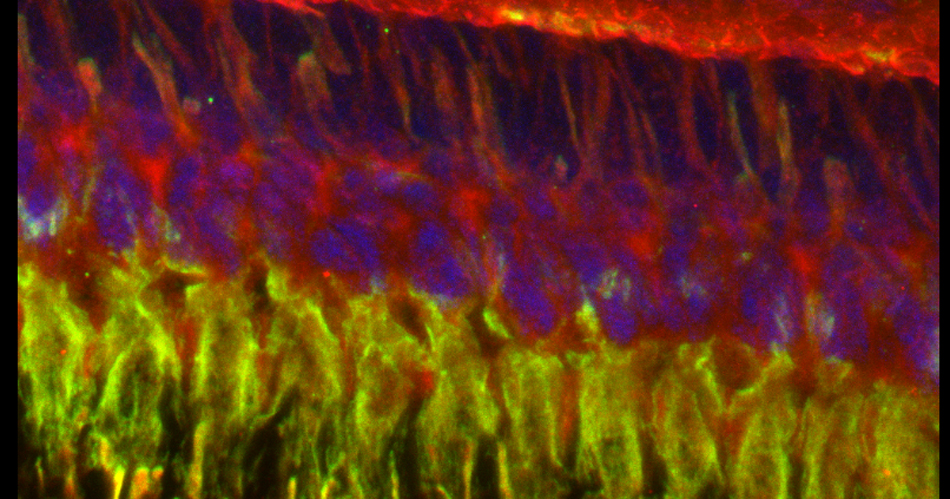
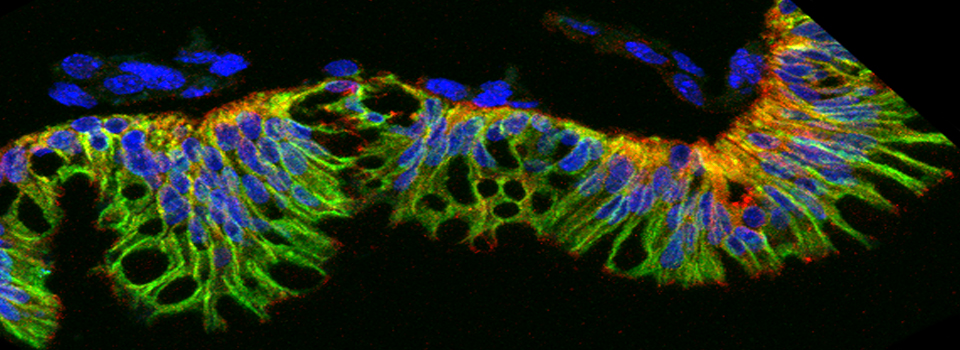
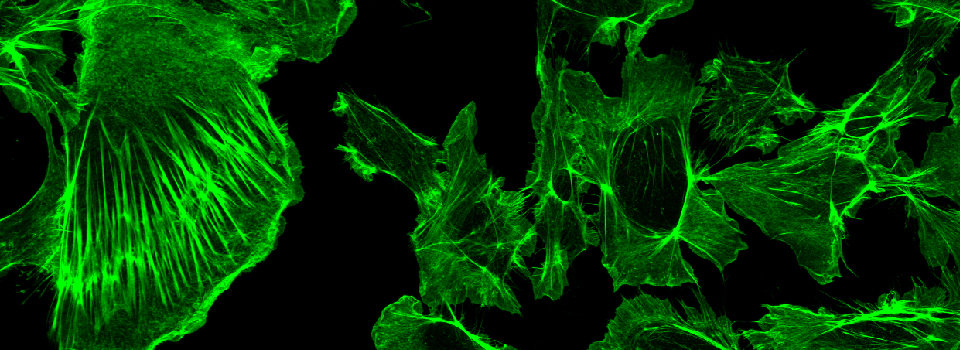
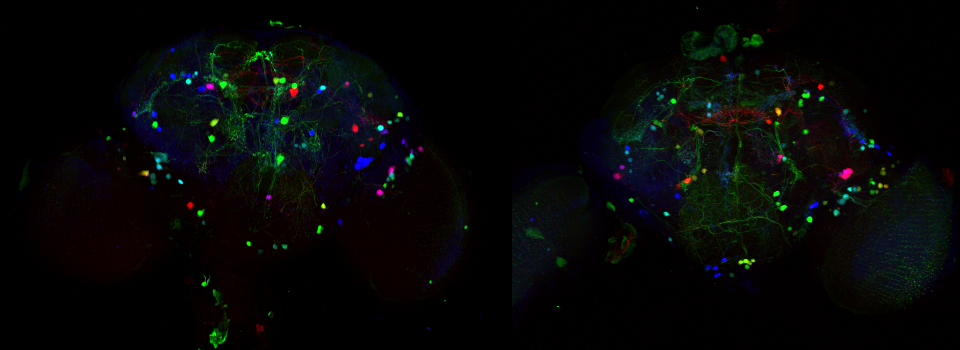
Images from our April 2018 FV3000 demo:
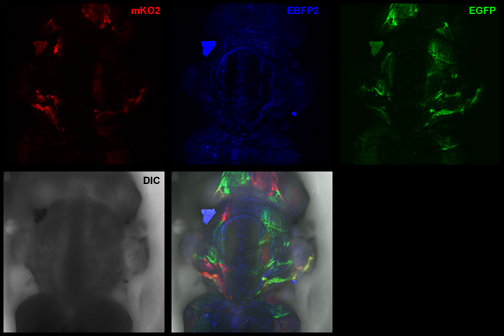
This BrainBow Drosophila pupa is expressing EBFP2/EGFP/mKO2 in regions of the cuticle derived from the dorsal half of the imaginal discs (DE-GAL driver). EBFP2 is a notoriously weak fluor, but the use of the GaASP detectors and the resonance scanner enables us to detect its signal in a live specimen.
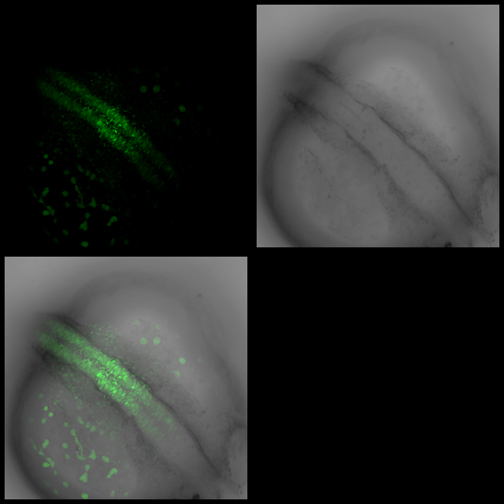
A zebrafish embryo expressing a gbx1-GFP reporter in the hindbrain.